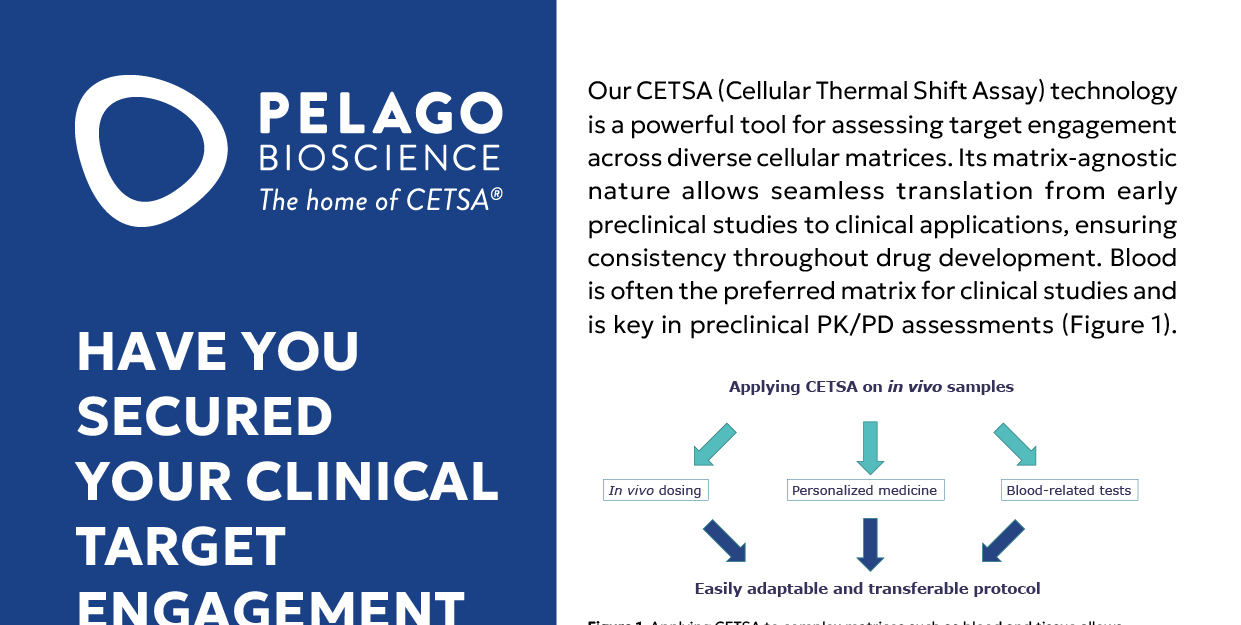
Validating target engagement is a crucial step in drug development, helping us understand how a drug interacts with its intended target and linking that interaction to its clinical effects. As therapies move from the lab to patient trials, having the right assay is essential—not just for confirming target engagement, but also for monitoring dosage and ensuring the drug reaches where it needs to go. Whole blood is often the preferred matrix in clinical studies because it reflects actual physiological conditions, but working with such a complex system comes with its own challenges.
CETSA is a Game-Changer in Drug Discovery and Development
At Pelago Bioscience, we use our CETSA® (Cellular Thermal Shift Assay) technology to provide precise, reliable data on target engagement across different biological environments. Because CETSA is matrix-agnostic, it seamlessly translates from early-stage drug discovery to clinical applications, including whole blood studies. This makes it an invaluable tool in pharmacokinetic (PK) and pharmacodynamic (PD) research, helping researchers confidently track drug activity throughout development.
The Role of CETSA in Whole Blood Target Engagement Studies
CETSA gives researchers a direct and physiologically relevant way to measure drug-target interactions in situ, considering factors like protein levels, compound uptake, and modifications. In collaboration with AbbVie, we developed a robust CETSA protocol to track RIPK1 target engagement in whole blood using AlphaLISA™ and MSD® detection methods. This work demonstrated that CETSA can assess target engagement in fresh and frozen blood samples—a critical feature for handling clinical samples requiring storage and transportation.
We also applied CETSA to study Akt inhibitors, first developing an assay in K562 cells before successfully adapting it for human whole blood. The results showed donor-specific differences in protein levels and melting behavior, confirming the assay’s ability to provide robust target engagement data.
A Scalable Approach to Clinical Target Engagement
Unlike traditional biochemical assays, CETSA directly measures target engagement in complex biological systems without modifying the drug or the target protein. Researchers can study drug behavior under real physiological conditions, providing insights that support better clinical decision-making.
Our workflow is designed to be scalable and efficient. It starts with protein detection in cell lines, adapts the assay for whole blood or PBMCs, and then generates melt and shift curves to confirm target engagement. This process ensures reproducible, high-quality data supporting a wide range of drug targets in clinical research.
With regulatory bodies increasingly prioritizing biomarker-driven drug development, having assays that deliver reliable and translatable target engagement data has never been more critical. CETSA helps bridge the gap between preclinical research and real-world clinical outcomes, enabling more informed drug dosing and development decisions.
Discover the Full Potential of CETSA in Clinical Research
Are you ready to secure your clinical target engagement assay? Discover how CETSA can accelerate your clinical research by downloading our application note.